Abstract 1. The role of nanotechnology in the development of power battery materials (Theroleofnanotechnologyinthedevelopmentofbatterymaterialsforelectr...
1. The role of nanotechnology in the development of power battery materials
(The role of nanotechnology in the development of battery materials for electric vehicles)
Both academia and industry are vigorously developing batteries to meet the needs of electric vehicles. In the process of designing and preparing electrode materials, nanotechnology-based methods have shown many advantages in improving energy density and power density, cycle stability and safety. Recently, Khalil Amine et al. of the Argonne National Laboratory in the United States reviewed the commercialization and commercialization of nanostructured materials for hybrid electric vehicles, and they discussed the development of materials to meet the needs of remote electric vehicles. (Nature Nanotechnology DOI: 10.1038/NNANO.2010.207)
2. Photocatalytic hydrogen production by hydroiodic acid
(Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution)

The decomposition of hydrogen by hydrogen-driven hydrogen halide is an important and rapidly developing research direction. In addition to hydrogen, the chemical produced (X2/X3−) is itself an important product. Park et al. reported a cost-effective and easily scalable method for photocatalytic decomposition of hydrogen iodide (HI) using MAPbI3 (methylammonium lead iodide). Considering that MAPbI3 is a water-soluble ionic compound, Park explored the equilibrium of precipitation and decomposition of MAPbI3 in saturated aqueous solutions. The concentration of I- and H+ in the aqueous solution is a key parameter for the stability of the tetragonal MAPbI3 phase. They demonstrate the stable and efficient hydrogen production under visible light. When Pt was used as a cocatalyst, the solar-driven HI decomposition efficiency was 0.81%. (Nature Energy DOI: 10.1038/NENERGY.2016.185)
3. Oxygen vacancies increase MoO3-x tantalum capacitor
(Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x)

Capacitor energy storage has the advantages of ultra-fast charging and high power, making it an attractive battery replacement. One limitation of electrochemical capacitors is their equal energy density. Therefore, the use of Faraday reactions to store charge in tantalum capacitor materials has attracted the interest of researchers. One of the tantalum capacitor materials is the orthorhombic MoO3 (α-MoO3), a layered compound with a high theoretical lithium capacity (279 mA hg−1 or 1,005 C g−1). Recently, Bruce Dunn of UCLA and his collaborators reported the tantalum capacitance of the reduced α-MoO3-x (R-MoO3-x) and compared the fully oxidized α-MoO3 (F-MoO3). The introduction of oxygen vacancies results in greater interlayer spacing, resulting in faster charging kinetics and more stable alpha-MoO3 structure during lithium ion insertion and extraction. The higher specific capacitance of R-MoO3-x is due to the reversible formation of a large amount of Mo4+. This study highlights the importance of potential when introducing oxygen vacancies in transition metal oxides and can be extended to other redox active materials. (Nature Materials DOI: 10.1038/NMAT4810)
4. Activity target of non-Pt group metal catalysts
(Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells)

The fuel cell is a zero-emission automotive power source with many advantages of a fuel car: low prepaid cost, long-range driving force and fast refueling. In order to make fuel cell vehicles a reality, the US Department of Energy (DOE) has set a fuel cell system with no cost target – $30 per kilowatt, removing several major powertrain components (a basic but complete internal combustion engine system is close to $3,000). ) equivalent to $2,400 per vehicle. To date, most research on fuel cell electric vehicles has focused on proton exchange membrane fuel cells (PEMFCs) because these systems require the highest power density. Recently, an alternative technology, hydroxide exchange membrane fuel cells (HEMFCs) has attracted the attention of researchers because it does not require Pt noble metals as a catalyst and has an inherent long-term cost advantage. Yan et al. reviewed the cost status of PEMFCs and the advantages of HEMFCs. In particular, they discussed the catalyst development required for HEMFCs and set an activity target that is comparable to current PEMFCs. To achieve these goals, nanostructures need to be carefully optimized to compress high specific surface areas to very small volumes while maintaining high area surface activity and efficient pore transport properties. (Nature Nanotechnology DOI: 10.1038/NNANO.2016.265)
5. Highly sensitive graphene-polymer nanocomposite sensor
(Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposite)

Despite the wide range of applications of nanocomposites, the incorporation of graphene into highly viscoelastic polymer precursors has not been well studied. Boland et al. added graphene to a slightly crosslinked silicone polymer, similar to a plasticine, radically altering the electrochemical properties of the polymer. The resulting nanocomposites exhibit extraordinary electrochemical behavior, such as a brief disappearance in the later stages of electrical resistance deformation and a non-monotonic change in electrical resistivity with strain. These phenomena are related to the movement of the nanosheets in the low viscosity polymer matrix. They also built quantitative models to describe electrochemical performance. These nanocomposites can be used as high-sensitivity (>500) electrochemical sensors to measure pulse and blood pressure, even the effects of a small spider's footsteps. (Science DOI: 10.1126/science.aag2879)
6. Using nanomaterials to design and develop scalable artificial photosynthesis technology
(Developing a scalable artificial photosynthesis technology through nanomaterials by design)

Artificial photosynthetic systems can directly utilize sunlight to generate fuel, thereby providing scalable energy storage methods and techniques for carbon neutral products for high energy density fuels. Various designs are currently being explored to produce viable artificial photosynthetic systems and state of the art semiconductor photoelectrode based technology systems. Lewis discussed a structural concept first proposed ten years ago, incorporating a semiconductor microwire array with a flexible polymer film. He highlighted key aspects of the ability to produce fully functional solar fuel generators, including the use of nanotechnology at all device manufacturing levels, including the discovery of abundant earth-based electrocatalysts for fuel formation and for stabilization. The material of the light absorber. He believes that this is safe, reliable, efficient and scalable for the other scientific and engineering challenges faced by artificial photosynthetic systems. (Nature Nanotechnology DOI: 10.1038/NNANO.2016.194)
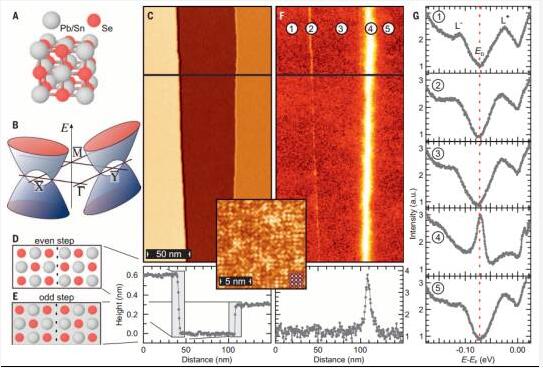
7. Spin-polarized intermediate band gap state at the edge of the topological crystalline insulator
(Robust spin-polarized midgap states at step edges of topological crystalline insulators)
Topological crystalline insulators are materials of the topological protective surface state with chiral spin texture caused by crystal symmetry, making it a potential candidate for spintronics applications. Sessi et al. used scanning tunneling to reveal the existence of a one-dimensional (1D) intermediate band gap state at the edge of the odd-atomic surface of a three-dimensional topologically crystalline insulator (Pb, Sn) Se. The minimum Toy model and the realistic tight-binding calculations determine that they are spin-polarized flats connecting the two Dirac points. This important reason provides a one-dimensional intermediate band gap state with inherent stability and protects them from backscattering. (Science DOI: 10.1126/science.aah6233)
8. Electrode doping of solution-based semiconducting polymer films
(Solution-based electrical doping of semiconducting polymer films over a limited depth)
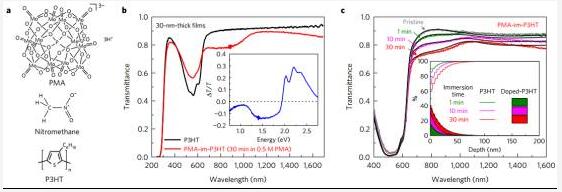
Solution-based electrical doping schemes can make the design of electronic devices more versatile; however, previous studies have shown that controlling the diffusion and stability of dopants in organic semiconductors remains challenging. Kolesov et al. proposed a solution-based method for p-type electrical doping of an electron-conjugated organic semiconductor thin film and its doping at a finite depth with a decay constant of 10-20 nm at the finite depth, ie by The post treatment is immersed in a nitromethane solution (phosphorous molybdate, PMA). The PMA doped film exhibits enhanced conductivity and work function, reduces solubility in processing solvents, and improves photooxidation stability in air. This method is applicable to various organic semiconductors used in photovoltaic and FETs. Incorporating an amine-containing polymer in a solution for film formation, the PMA doping at a limited depth of the bulk heterojunction polymer film enables a single-layer organic photovoltaic device that can be processed at room temperature to have a power conversion of up to 5.9 ± 0.2%. Efficiency and stability for at least 280 hours at 60 °C. (Nature Materials DOI: 10.1038/NMAT4818)
This article is authorized by the new material online (micro signal: xincailiaozaixian), if other media need to reprint, please contact the new material online small series (micro signal) 4 Blades Drill Bits
Metal Drill Bit Set,Plate Drag Drill Bits,Anchor Nut Drill Bits,4 Blades Drill Bits
J.B Machinery (Ningbo) Co., Ltd. , https://www.jbdrill.com