Progress in Research on Electrocatalytic Hydrogen Evolution of Carbon Materials in China University of Science and Technology
In recent years, hydrogen production from electrolyzed water has attracted widespread attention in the academic community. Finding inexpensive and efficient non-platinum electrocatalysts has become a hot topic of research. As the current "star material" graphene has the advantages of good conductivity, corrosion resistance, etc., researchers are committed to develop it as a high activity acidic hydrogen evolution electrocatalyst, but many of the carbon-based catalyst activity is very different from the precious metal, How to develop graphene carbon-based materials into high-activity electrocatalysts is a hot topic. Recently, Professor Chen Qianwang, Professor of Material Research Center of Hefei National University of Science and Technology, China University of Science and Technology, and Department of Materials, School of Chemistry and Materials Science, used noble metal gallium-doped metal-organic framework materials as precursors to prepare nitrogen-doped one-step calcined materials. The graphene layer enwraps the samarium-cobalt alloy core-shell structure material and exhibits high activity and high stability in the hydrogen evolution reaction of acidic electrolytes. Related research results are published in "Advanced Materials". The samarium-cobalt alloy can transfer electrons to the surface active sites. The nitrogen-doped graphene layer coated on the surface of the samarium cobalt alloy resembles “shell armorâ€, which helps to prevent the alloy core from being corroded by the acid. As an acidic hydrogen evolution electrocatalyst (having a niobium content of only 1.56 wt.%), its Tafel slope is only 23 mV/dec, and its overpotential at a current density of 10 mA/cm2 is only 24 mV, showing 20% ​​of commercial use. Comparable electrocatalytic hydrogen evolution performance of Pt/C electrocatalysts. Density functional theory simulation calculations found that the adjacent carbon atom of nitrogen-doped atoms is the active site of the electrocatalytic reaction. The introduction of ruthenium promotes the migration of electrons to the surface of the graphene-like layer, and reduces the hydrogen adsorption free energy of the active site. The surface structure characterization of the material and the imaging analysis of the elemental composition have revealed that the increase in the doping amount of nitrogen and the enrichment of niobium at the inner surface of the alloy contribute to the improvement of the catalyst performance. This work provides new ideas for finding cheaper and more efficient electrocatalytic hydrogen evolution catalysts in the future. Research work has been supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Central Research Institutions of the Ministry of Education. Mixer Faucet,Lab Mixer Faucet,Laboratory Mixer Faucet,Single Handle Mixer Faucet NARWILL IMPORT&EXPORT CO.LTD , https://www.narwill.com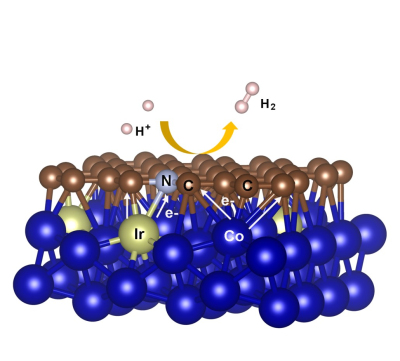
Catalyst structure and electrocatalytic hydrogen evolution reaction